Dedicated Regulatory Team
Depending on its Outstanding Regulatory Team, JMS aims to provide leadership, guidance, and expertise to its local and international partners; including clinical development, medical affairs, manufacturing, and commercial marketing on regulatory matters throughout the life cycle of a drug. Further, JMS Regulatory Team was established to work closely with both pharma industry stakeholders and regulatory bodies to have an impact on governance and decision making.
JMS Regulatory Team is dedicated to make a significative distinction to patients’ health, based on utilizing the profound scientific knowledge and the latest innovative sciences and technologies to the search for new medications for Arab peoples. We are aware of commercial need of medicinal products to develop the business as well as we are dedicated to register products to the market at the earliest opportunity.
The excellence of JMS Regulatory Team relies typically on the following four key competencies that are indispensable to fulfill its mission:
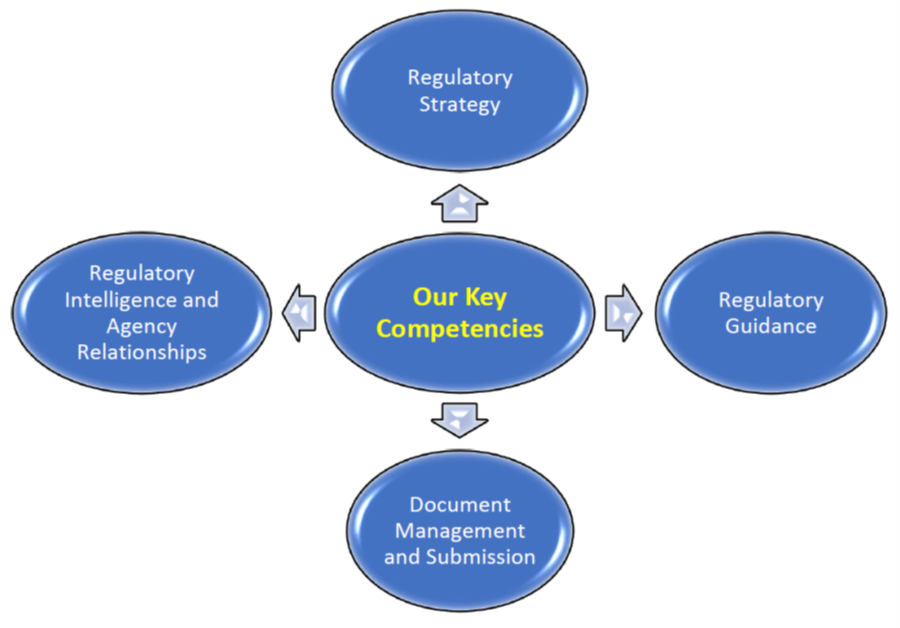
Our main objectives are:
-
o
- To provide customer satisfaction by providing expert and specialized knowledge. o
- To seize the opportunity when available and to be creative in all that we do. o
- To deliver quality, based on implementing proactive processes rather than reactive. o
- To add value by exploring innovative and most effective ways of sharing knowledge through training and learning from our successes and failures.
JMS Regulatory Team is your reliable partner for regulatory, scientific and medical services.


JAMJOOM
MEDICINE
STORE